| Product Number | 15-1382 |
| CAS # | 874948-63-7 |
| Molecular Formula | C50H57O4P |
| Formula Weight | 753.0 |
| Purity | 98%, (99% ee) |
| Safety | Yes |
Technical Notes:
1.Pictet-Spengler Reaction: Catalyst for the asymmetric Pictet-Spengler reaction, where substituted
tryptamines are treated with an aldehyde in the presence of a catalytic amount of a chiral phos phoric acid.
2.Spiroketalization: The chiral catalyst can override the inherent preference for the formation of thermodynamic spiroketals, and highly selective formation of nonthermodynamic spiroketals could be achieved under the reaction conditions
3.oxa-Diels-Alder Cycloaddition: An asymmetric cascade annulation between 2-hydroxystyrenes and 2- alkynylbenaldehyes or 1 -(2-alkynylphenyl)ketones has been established with good to excellent
enantioselectivities, on the basis of an enantios elective oxa-Diels-Alder cycloaddition of in situ generated me tallo-isochromenylium intermediates, by cooperative binary catalysis of Pd(OAc)2 and (S)-TRIP
4.Hydrogenation: A 1 mol % loading of the chiral phosphoric acid catalyst converts aromatic and aliphatic
imines such as into the corresponding amines in high yields and enantioselectivities if treated with Hantzschdihydropyridine
5.Kinetic Resolution: An efficient and simple protocol for the kinetic resolution of secondary alcohols. The system is based on a combination of chiral Bronsted acid, DABCO, and acetyl chloride to gives various enantioenriched alcohols with selectivity factors up to 105.
![(11bS)-4-Hydroxy-2,6-bis[2,4,6-tris(1-methylethyl)phenyl]-4-oxide-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin, 98%, (99% ee)](http://www.jk-sci.com/cdn/shop/files/807454_150x150_crop_center.png?v=1761106266)
![(11bS)-4-Hydroxy-2,6-bis[2,4,6-tris(1-methylethyl)phenyl]-4-oxide-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin, 98%, (99% ee)](http://www.jk-sci.com/cdn/shop/files/807454_600x600_crop_center.png?v=1761106266)


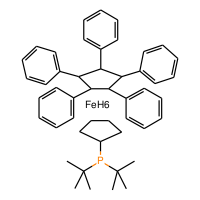
![(R)-(+)-1-[(R)-2-(2'-Di-3,5-xylylphosphinophenyl)ferrocenyl]ethyldi-3,5-xylylphosphine, min. 97%](http://www.jk-sci.com/cdn/shop/files/GeneralChemical_optimized_241e3f17-1e81-436d-8544-207409998e58_200x200_crop_center.png?v=1761107494)
![(R)-(+)-1-[(R)-2-(2'-Diphenylphosphinophenyl)ferrocenyl]ethyldiphenylphosphine, min. 97%](http://www.jk-sci.com/cdn/shop/files/GeneralChemical_optimized_0bcf0905-e50c-490f-accb-0bb5f8f7675c_200x200_crop_center.png?v=1761107487)
![(R)-(-)-1-[(R)-2-(2'-Diphenylphosphinophenyl)ferrocenyl]ethyldicyclohexylphosphine, min. 97%](http://www.jk-sci.com/cdn/shop/files/GeneralChemical_optimized_a9c387d3-8ace-4f00-8a1b-9ee787c1f394_200x200_crop_center.png?v=1761107484)
![(R)-(-)-1-[(R)-2-(2'-Diphenylphosphinophenyl)ferrocenyl]ethylbis(di-3,5-trifluoromethylphenyl)phosphine, min. 97%](http://www.jk-sci.com/cdn/shop/files/GeneralChemical_optimized_2232f6aa-b105-4bf9-af2b-d7a839a3dbab_200x200_crop_center.png?v=1761107481)
