The Wagner-Meerwein rearrangement uses catalytic acid to convert an alcohol into an olefin. The alcohol is protonated and released as water to form a carbocation. A [1,2]-shift of an adjacent carbon-carbon bond generates a more stable carbocation, followed by loss of a proton to afford the alkene. Both E/Z products...

The Williamson ether synthesis is an SN2 reaction The Williamson ether synthesis uses a base and an alkyl halide to convert an alcohol into a symmetrical or an unsymmetrical ether. The base deprotonates the alcohol to form an alkoxide that undergoes a nucleophilic substitution (SN2) reaction with the alkyl halide,...

The Wittig reaction uses triphenylphosphine (PPh3), a base, and a 1° or 2° alkyl halide to convert an aldehyde or ketone to an olefin. First a phosphonium salt is made when the PPh3 attacks the alkyl halide, releasing a halide ion in the process. The α-hydrogen is then deprotonated by...
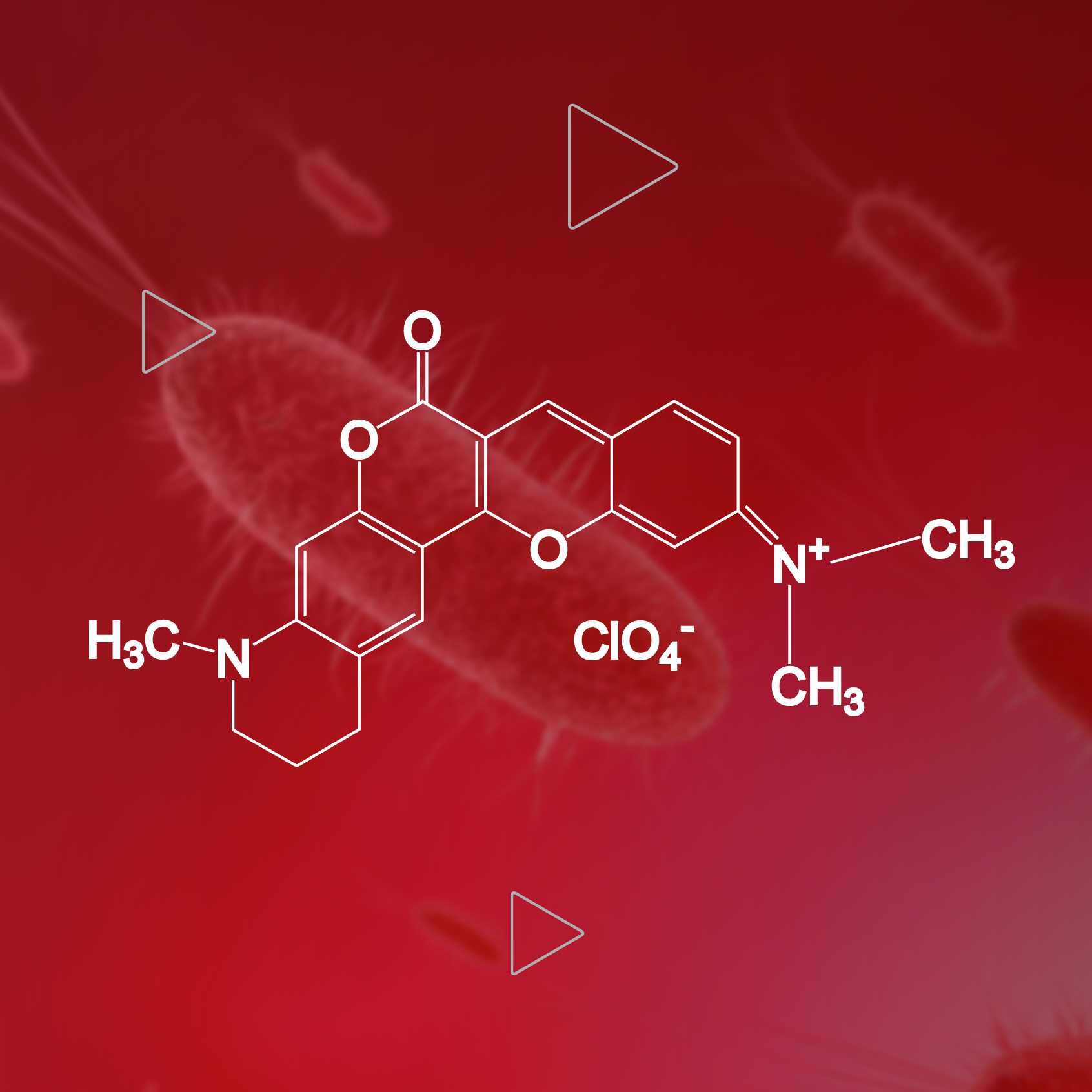
RNA molecules have various applications in living cells, such as 1) transmission and translation of genetic information, 2) structural support of molecular machines, 3) regulation of gene expression, silencing and catalysis. Obtaining a complete spatiotemporal distribution of RNA synthesis, processing, and transportation is essential for understanding the function and behavior...

The Wolff-Kishner reduction uses hydrazine, a base, and a high-boiling solvent to convert an aldehyde or ketone to an alkane. The hydrazine first attacks the ketone or aldehyde, releasing water to form a hydrazone intermediate. Subsequent proton transfer steps result in the release of nitrogen gas and the formation of...

The Wolff rearrangement uses either catalytic silver oxide, heat, or light to convert an α-diazo ketone to a ketene. In a one-step reaction, the catalyst initiates a 1,2-shift to release nitrogen gas and produce ketene. Due to the release of nitrogen gas, this reaction should not be performed in a...

